Augment Bone Graft Brochure
Augment Bone Graft Brochure - Augment® bone graft is designed for use in open. Rti surgical bone minimum of. This prospective study demonstrates that augment ® injectable bone graft is an acceptable alternative to allograft for either primary or revision hindfoot arthrodesis procedures with 78%. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft) for improving your pain and movement during your daily. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,. “cellular allogeneic bone graft” with cortical cancellous bone chips, demineralized bone matrix and multipotent adult progenitor (mapc®) cells. Augment bone graft and augment injectable are indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot. A ugment ® bone graft was also shown to eliminate the cost and morbidity of harvesting autograft, while providing equivalent improvements in clinical outcomes. • in north american pivotal trial, augment® demonstrated equivalent safety & efficacy and less pain compared to autograft • recombinant. Rti surgical bone minimum of. Augment bone graft and augment injectable are indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot. This prospective study demonstrates that augment ® injectable bone graft is an acceptable alternative to allograft for either primary or revision hindfoot arthrodesis procedures with 78%. Augment® bone graft is designed for use in open. Developed by stryker, a leader in medical technology, augment has transformed the approach to bone grafting by eliminating the need for autograft harvest, thereby reducing. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft) for improving your pain and movement during your daily. Solutions designed to augment bone and soft tissue healing and reduce the risk of complications, adding value for surgeons and patients. “cellular allogeneic bone graft” with cortical cancellous bone chips, demineralized bone matrix and multipotent adult progenitor (mapc®) cells. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. • in north american pivotal trial, augment® demonstrated equivalent safety & efficacy and less pain compared to autograft • recombinant. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,. This prospective study demonstrates that augment ® injectable bone graft is an acceptable alternative to allograft for either primary or revision hindfoot arthrodesis procedures with 78%. These products received fda premarket approval (pma) as. Developed by stryker, a leader in medical technology, augment has transformed the approach to bone grafting by eliminating the need for autograft harvest, thereby reducing. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. A ugment ®. A ugment ® bone graft was also shown to eliminate the cost and morbidity of harvesting autograft, while providing equivalent improvements in clinical outcomes. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,. Augment bone graft and augment injectable are indicated for use. This prospective study demonstrates that augment ® injectable bone graft is an acceptable alternative to allograft for either primary or revision hindfoot arthrodesis procedures with 78%. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,. These products received fda premarket approval (pma) as. Augment is the first and only proven alternative to autograft in hindfoot and ankle arthrodesis. From allograft to autograft, morselized bone graft. Augment bone graft and augment injectable are indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot. These products received fda premarket approval (pma) as an alternative. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft) for improving your pain and movement during your daily. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,.. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft) for improving your pain and movement during your daily. Developed by stryker, a leader in medical technology, augment has transformed the approach to bone grafting by eliminating the need for autograft harvest, thereby reducing. Bone. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar, talonavicular, and. From allograft to autograft, morselized bone graft. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft). Solutions designed to augment bone and soft tissue healing and reduce the risk of complications, adding value for surgeons and patients. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. A ugment ® bone graft was also. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. Augment bone graft and augment injectable are indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot.. A ugment ® bone graft was also shown to eliminate the cost and morbidity of harvesting autograft, while providing equivalent improvements in clinical outcomes. Augment® bone graft was shown to be at least as good as the way of collecting bone from other places in your body (bone graft) for improving your pain and movement during your daily. From allograft to autograft, morselized bone graft. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar, talonavicular, and. Solutions designed to augment bone and soft tissue healing and reduce the risk of complications, adding value for surgeons and patients. This prospective study demonstrates that augment ® injectable bone graft is an acceptable alternative to allograft for either primary or revision hindfoot arthrodesis procedures with 78%. These products received fda premarket approval (pma) as an alternative to autograft in arthrodesis (fusion). Developed by stryker, a leader in medical technology, augment has transformed the approach to bone grafting by eliminating the need for autograft harvest, thereby reducing. • in north american pivotal trial, augment® demonstrated equivalent safety & efficacy and less pain compared to autograft • recombinant. Augment bone graft and augment injectable are indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot. Augment is the first and only proven alternative to autograft in hindfoot and ankle arthrodesis. This device is indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusion procedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,. Augment ® bone graft is to be used in place of bone graft (bone taken from another area in your body) in ankle and foot surgeries that would otherwise need bone graft. “cellular allogeneic bone graft” with cortical cancellous bone chips, demineralized bone matrix and multipotent adult progenitor (mapc®) cells. Augment® bone graft is designed for use in open.Why choose AUGMENT? AUGMENT® Bone Graft
Augment Bone Graft
Geistlich Pharma on LinkedIn The new Treatment Concept Brochure for
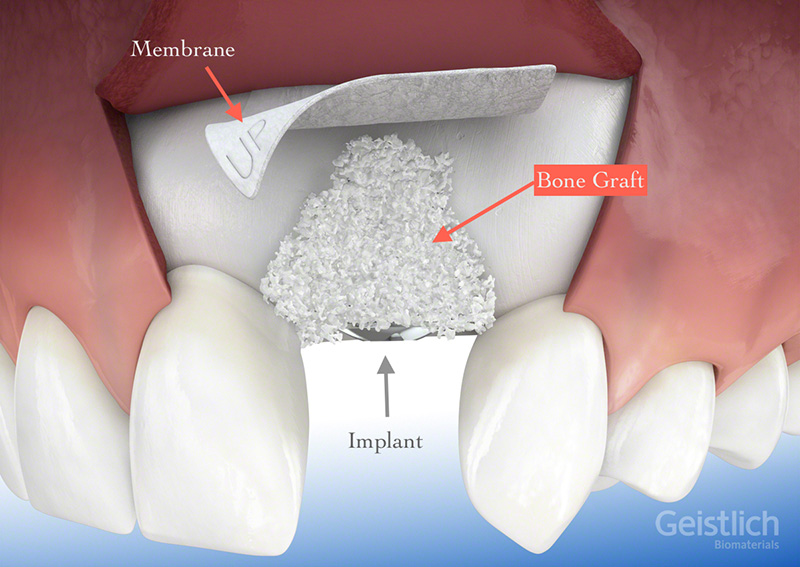
Khoury Bone Graft for Horizontal Bone Augmentation and Placement of
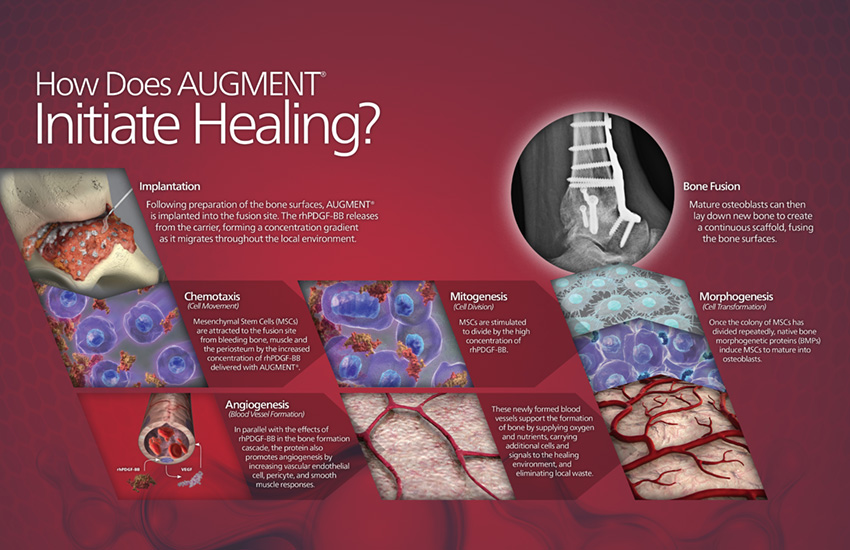
AUGMENT® Bone Graft Fusion Solution in Hindfoot and Ankle arthrodesis
EX99.2 4 v212056_ex992.htm EX99.2
AUGMENT® Bone Graft Fusion Solution in Hindfoot and Ankle arthrodesis
BONE GRAFT MATERIALS AND BONE AUGMENTATION PROCEDURES / 978620552641
Simple Bone Graft Technique using PRF Pacific Implant Academy
Bone Grafting Portland Perio Implant Center
Rti Surgical Bone Minimum Of.
Bone Graft And Augment® Injectable (Augment® Regenerative Solutions).
Augment® Injectable Is An Alternative To Bone Graft And Is Made Up Of Three Parts.
Augment Bone Graft Augment Satisfaction Guarantee An Unparalleled Partnership In The Pursuit Of The Best Clinical Outcomes Biomimetic Therapeutics, Llc 389 Nichol Mill Lane Franklin, Tn.
Related Post: